你现在所在位置:资讯首页 > 专题讲座
Nature:脂质怎样影响复杂膜结构?
医学资讯 发布时间:2014-06-08 点击:最近发表的很多高分辨率膜蛋白结构都存在与蛋白密切相关的脂质,这促使人们提出一个问题:这些脂质是怎样影响复杂的膜结构的?
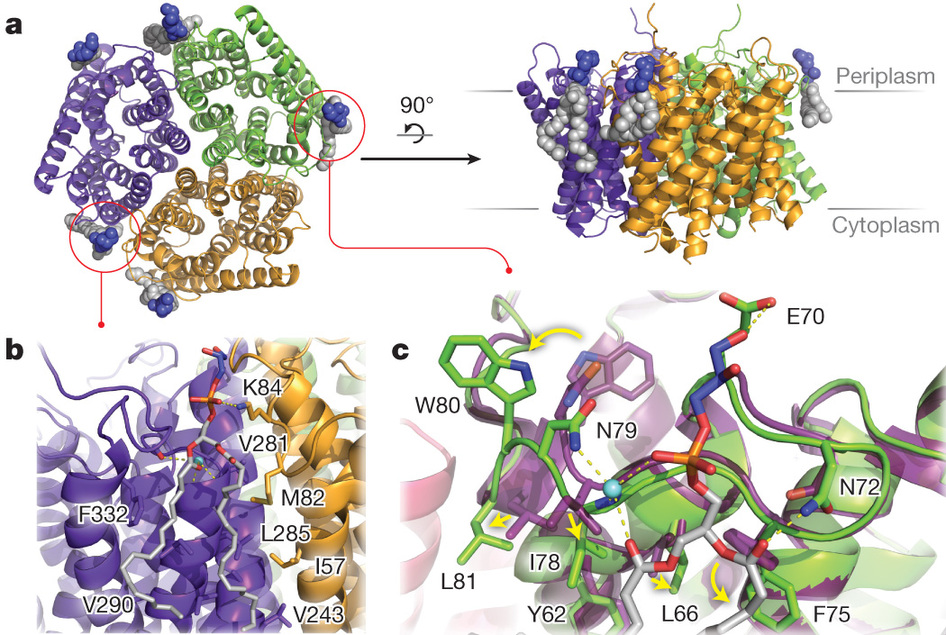
Carol Robinson及同事建立了一个新的离子迁移质谱(IM-MS)方法,它使其能够获得与脂质结合在一起的折叠的蛋白构形的高分辨率谱。
利用这一方法,他们识别出改变了MscL (具有大电导率的机械敏感性通道)、水通道蛋白-Z和氨通道的稳定性的脂质。然后,他们还确定了这些脂质当中与氨通道结合在一起的一个脂质(磷脂酰甘油)的X-射线晶体结构,该结构显示了某一特定环中的一个构形变化是怎样导致一个磷脂酰甘油结合点之形成的。
这项研究的主要结论是,一次脂质结合事件能改变一个膜复合物的稳定性。
原文链接:Membrane proteins bind lipids selectively to modulate their structure and function
Abstract:Previous studies have established that the folding, structure and function of membrane proteins are influenced by their lipid environments and that lipids can bind to specific sites, for example, in potassium channels8. Fundamental questions remain however regarding the extent of membrane protein selectivity towards lipids. Here we report a mass spectrometry approach designed to determine the selectivity of lipid binding to membrane protein complexes. We investigate the mechanosensitive channel of large conductance (MscL) from Mycobacterium tuberculosis and aquaporin Z (AqpZ) and the ammonia channel (AmtB) from Escherichia coli, using ion mobility mass spectrometry (IM-MS), which reports gas-phase collision cross-sections. We demonstrate that folded conformations of membrane protein complexes can exist in the gas phase. By resolving lipid-bound states, we then rank bound lipids on the basis of their ability to resist gas phase unfolding and thereby stabilize membrane protein structure. Lipids bind non-selectively and with high avidity to MscL, all imparting comparable stability; however, the highest-ranking lipid is phosphatidylinositol phosphate, in line with its proposed functional role in mechanosensation9. AqpZ is also stabilized by many lipids, with cardiolipin imparting the most significant resistance to unfolding. Subsequently, through functional assays we show that cardiolipin modulates AqpZ function. Similar experiments identify AmtB as being highly selective for phosphatidylglycerol, prompting us to obtain an X-ray structure in this lipid membrane-like environment. The 2.3 Å resolution structure, when compared with others obtained without lipid bound, reveals distinct conformational changes that re-position AmtB residues to interact with the lipid bilayer. Our results demonstrate that resistance to unfolding correlates with specific lipid-binding events, enabling a distinction to be made between lipids that merely bind from those that modulate membrane protein structure and/or function. We anticipate that these findings will be important not only for defining the selectivity of membrane proteins towards lipids, but also for understanding the role of lipids in modulating protein function or drug binding.
相关文章

高脂肪饮食或可延缓脑衰老一提到高脂肪饮食,人们第一之间往往想到的是肥胖,接下来就一...