你现在所在位置:资讯首页 > 专题讲座
Nature:磷酸化的泛素是“泊蛋白”的一个活化因子
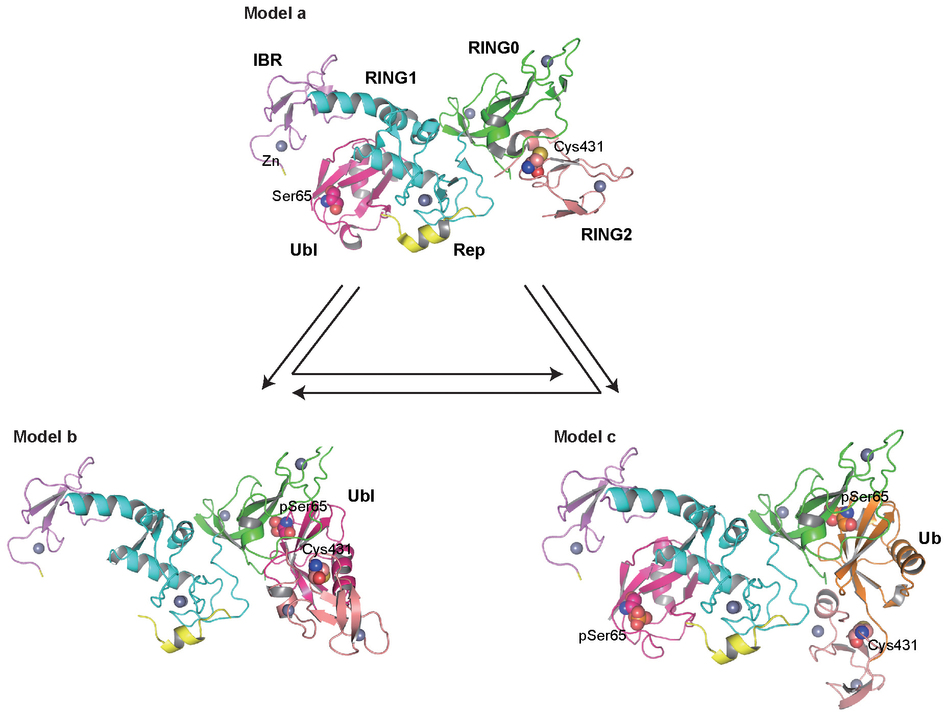
医学资讯 发布时间:2014-06-07 点击:小蛋白“泛素”(以其通过与其他蛋白相结合和调控它们的活性或稳定性来在这些蛋白的转录后修饰中所起作用而为人们所熟悉)在这项研究中被发现是激酶PINK1的基质,后者与泛素连接酶“泊蛋白”(parkin)一起是隐性遗传性帕金森氏症的一个致病基因。Noriyuki Matsuda及同事发现,在线粒体膜电位降低之后,PINK1 在“丝氨酸残基65”上将泛素磷酸化,然后被磷酸化的泛素与也被PINK1磷酸化的“泊蛋白”发生相互作用。这种相互作用使得“泊蛋白”的酶活性能够被完全激活,后者涉及用泛素标记线粒体基质。
原文链接:Ubiquitin is phosphorylated by PINK1 to activate parkin
Abstract:PINK1 (PTEN induced putative kinase 1) and PARKIN (also known as PARK2) have been identified as the causal genes responsible for hereditary recessive early-onset Parkinsonism1, 2. PINK1 is a Ser/Thr kinase that specifically accumulates on depolarized mitochondria, whereas parkin is an E3 ubiquitin ligase that catalyses ubiquitin transfer to mitochondrial substrates3, 4, 5. PINK1 acts as an upstream factor for parkin6, 7 and is essential both for the activation of latent E3 parkin activity8 and for recruiting parkin onto depolarized mitochondria8, 9, 10, 11, 12. Recently, mechanistic insights into mitochondrial quality control mediated by PINK1 and parkin have been revealed3, 4, 5, and PINK1-dependent phosphorylation of parkin has been reported13, 14, 15. However, the requirement of PINK1 for parkin activation was not bypassed by phosphomimetic parkin mutation15, and how PINK1 accelerates the E3 activity of parkin on damaged mitochondria is still obscure. Here we report that ubiquitin is the genuine substrate of PINK1. PINK1 phosphorylated ubiquitin at Ser 65 both in vitro and in cells, and a Ser 65 phosphopeptide derived from endogenous ubiquitin was only detected in cells in the presence of PINK1 and following a decrease in mitochondrial membrane potential. Unexpectedly, phosphomimetic ubiquitin bypassed PINK1-dependent activation of a phosphomimetic parkin mutant in cells. Furthermore, phosphomimetic ubiquitin accelerates discharge of the thioester conjugate formed by UBCH7 (also known as UBE2L3) and ubiquitin (UBCH7~ubiquitin) in the presence of parkin in vitro, indicating that it acts allosterically. The phosphorylation-dependent interaction between ubiquitin and parkin suggests that phosphorylated ubiquitin unlocks autoinhibition of the catalytic cysteine. Our results show that PINK1-dependent phosphorylation of both parkin and ubiquitin is sufficient for full activation of parkin E3 activity. These findings demonstrate that phosphorylated ubiquitin is a parkin activator.
相关文章

高脂肪饮食或可延缓脑衰老一提到高脂肪饮食,人们第一之间往往想到的是肥胖,接下来就一...